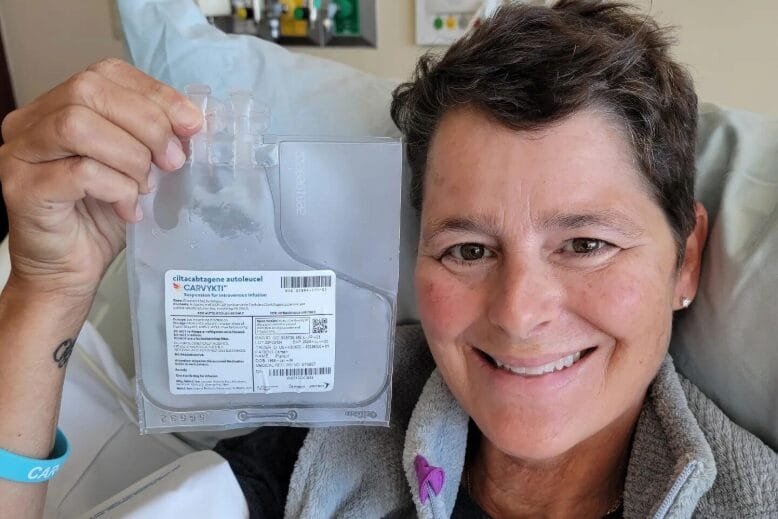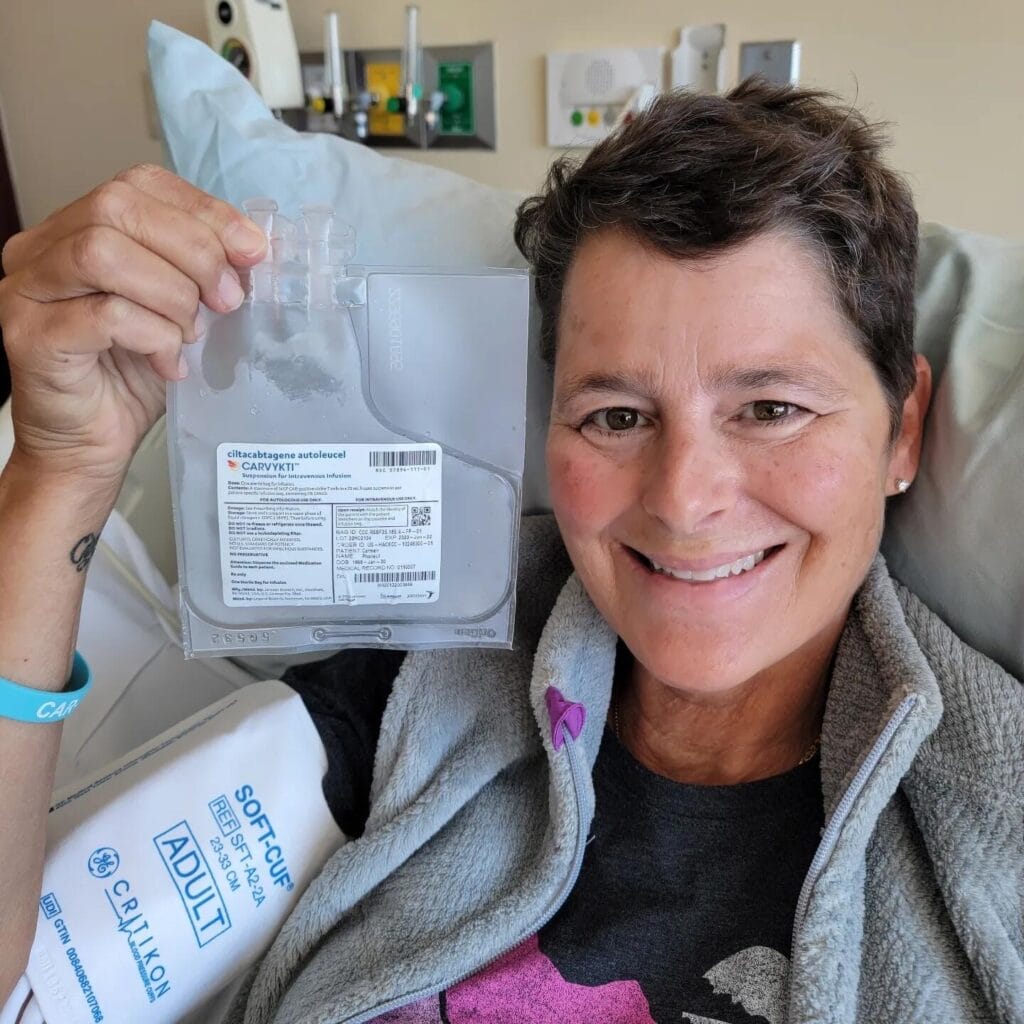

Carmen Phaneuf battled multiple myeloma for years until she tried a new treatment developed by a New Jersey biotech firm. Photo: Courtesy of Carmen Phaneuf
A groundbreaking treatment developed by a New Jersey-based company is giving hope to patients diagnosed with multiple myeloma, a rare and deadly blood cancer.
Carmen Phaneuf of Little Silver was diagnosed with the disease in 2002. Until recently, it had been generally considered incurable. A nurse practitioner at the Parker Family Health Center in Red Bank, she understood only too well what living with this medical diagnosis would mean. Since undergoing the treatment therapy known as Carvykti at Hackensack University Medical Center in November 2022, she has been cancer-free for three years.
“I’ve been living with this for 23 years, which is a very long time for this cancer,” she says. “This treatment has given me so much hope for the future. I’m ecstatic for the quality of life that it’s given me. The ability to live without chemotherapy, which has been really hard on my body for more than 10 years, is amazing.”
Phaneuf was diagnosed after a routine blood test found that she had a low white blood cell count. Until recently, she survived because of repeated chemotherapy treatments and steroids, which have been very taxing on her body. “I was always feeling badly from the treatment and feeling manic from the steroids that always accompanied my treatment.”
She is one of hundreds of New Jersey patients so far to have been given a one-time infusion of Carvykti, developed by Somerset-based Legend Biotech.
*
Multiple myeloma is a rare type of blood cancer diagnosed in less than 1 percent of people. It affects plasma B cells, which are white blood cells that produce antibodies. The cancerous cells accumulate in the bone marrow, crowding out healthy cells and causing various symptoms, including bone pain, anemia, kidney problems, fractures and heart failure. The disease weakens the immune system and is typically fatal.
There are other treatments on the market, but with those, patients usually have to cycle through various therapies and typically relapse after a few years, eventually succumbing to the disease.
This new treatment by Legend Biotech is remarkable because patients who have taken it through a study were found to be cancer-free for more than five years—typically considered a cure in the world of cancer treatment. The treatment, which manipulates a patient’s immune system into attacking the cancer cells, was approved by the U.S. Food and Drug Administration in 2022. Carvykti is a CAR T-cell therapy; it’s a personalized form of immunotherapy that trains your own immune cells to recognize and destroy cancer. (There is another CAR T-cell therapy that has been FDA-approved for the treatment of multiple myeloma, called Abecma, which is manufactured by Bristol Myers Squibb, which has offices in Summit).
Legend Biotech was founded as an early-stage cell-therapy company in 2014. The company was the brainchild of scientists who recognized that antibody-based therapeutics could potentially treat previously incurable diseases such as multiple myeloma.
Legend and its partner, Johnson & Johnson, which is helping to develop the treatment, started the trial with the Carvykti infusion in 2018 with 97 patients. They were thrilled with the results, says Ying Huang, the company’s chief executive officer. More than 8,000 patients so far have been treated with Carvykti around the world, he says.
“When we started the trial, we thought maybe we could prolong the survival rate for patients from one year to maybe two or three years. But we did not expect that these patients would survive for five years,” he says. “What’s also important is that this is a one-time treatment, and patients don’t have to keep coming back for treatments.”
The findings of the trial were presented at the American Society of Clinical Oncology’s annual meeting in June.
*
When Phaneuf was interviewed in early September, she was about to board a cruise to Alaska with her husband. An adventure traveler, she loves to ski, bike and take part in triathlons. Until recently, she managed the disease with chemotherapy and stem cell transplants. She usually had to start the regimen all over again every two years after relapsing. And the treatments were three weeks on and one week off, which was hard on her quality of life, she says.
“Skiing is my passion; I’ve skied all over the world. I’m really active—but the treatments were hard on my body,” she says.
Phaneuf read about the positive results that patients were getting from Carvykti, and that they didn’t need chemotherapy or maintenance medication after the treatment, so she talked to her doctor about it.
“I needed a break from chemotherapy. I wanted to take a chance to do this and just be able to live for a few years without chemo,” she says.
After her treatment with Carvykti, it took nine months to get her tumor-marker numbers to zero. “But since then, I have been in a complete remission,” she says. “It’s been great. I’m able to travel, like this trip to Alaska. It’s given me a lot of hope for the future. I’m ecstatic about the quality of life that it’s given me.”


Photo: Courtesy of Carmen Phaneuf
Phaneuf says she was aware of side effects related to the treatment, but felt it was worth the risk. “We need to put our trust in the science and in our health care providers. I would do it again for sure, and I will do it again, if and when this treatment stops working.”
*
While historically, multiple myeloma was deemed an incurable disease, physicians are now optimistic Carvykti may be able to successfully treat patients long-term.
Dr. Noa Biran, co-chief of the multiple myeloma division at John Theurer Cancer Center at Hackensack University Medical Center, has given hundreds of patients this treatment and says the results have been remarkable.
“The benefit of this treatment, and what makes it unique, is that patients historically needed to be on a maintenance or chronic therapy for a long time, but this treatment offers the possibility to come off the medication, because it’s a one-and-done approach,” she says. “Many, many patients are seeing long-term remissions without needing therapies, and it’s lasting a number of years.” While she doesn’t consider this treatment a complete cure yet, she does see it as a functional cure, meaning that patients who receive it will live so long that they will eventually die of something unrelated.
“This is a practice-changing treatment. It changed the bar for what we expect for response rates in relapsed myeloma. Historically, therapies have been approved based on 30 percent response rates, and we’re getting near a hundred-percent response rate to this treatment,” she says, adding, “This is a big deal. It’s very hard to find treatments that work in almost all cases.”
While the cost of the treatment is high—about $500,000—so far, most insurance companies have been covering it, as well as Medicare and Medicaid, says Huang. As a one-and-done treatment, Carvykti could even be more cost-effective than other treatments that extend for years. Other drugs to treat this disease can cost more than $300,000 per year, he says, “so our drug can actually save money.”
New Jersey patients seeking this treatment are fortunate to have locations in-state, including Hackensack University Medical Center, Rutgers Cancer Institute of New Jersey and Robert Wood Johnson University Hospital.
It’s especially convenient considering that patients have to spend about two weeks in the hospital being monitored for side effects after treatment.
Biran, who treated Phaneuf, says that while her patient may eventually need another treatment for her multiple myeloma, Phaneuf can be optimistic that by then, there might be a whole host of new treatments available.
“There are so many drugs on the horizon that I would be optimistic if and when the time comes,” she says.
CAR T-cell products are already being used successfully to treat other forms of blood cancers, such as leukemia and lymphoma, she says. Physicians are also looking to use this treatment for solid tumors, such as colon cancer and lung cancer. “We’re already treating patients in our hospital with other kinds of tumors, and it’s been successful,” Biran says.